
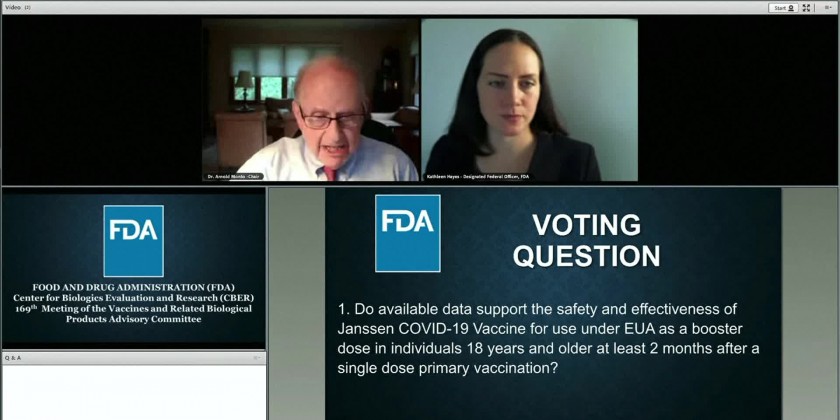
A panel of U.S. health advisers endorsed booster doses of Johnson & Johnson's single-shot COVID-19 vaccine.
J&J has asked the Food and Drug Administration for flexibility with its booster, arguing the extra dose adds important protection as early as two months after initial vaccination, but that it might work better if people wait until six months later.
Last month, booster doses of Pfizer's vaccine began for people at high risk of COVID-19, and the FDA advisory panel has recommended the same approach for Moderna recipients.
MITV is a broadcast TV brand intended for international and local English speaking consumers, launched on 31st March 2010 based in Yangon.